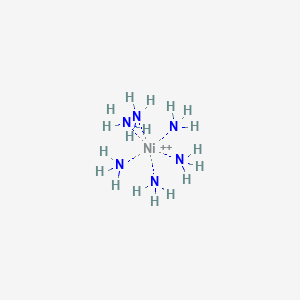
![Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1182/0*DWO_1XXK-IxUiSTt.jpg)
Why is [Ni (NH3)6]2+paramagnetic while [Ni (CN) 6]4-diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex. Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex.](https://haygot.s3.amazonaws.com/questions/1048239_879440_ans_9590dd8f7eff475e873fdfa26f547f7a.PNG)
Explain [Co(NH3)6]^3 + is an inner orbital complex whereas [Ni(NH3)6]^2 + is an outer orbital complex.
1. Details of Module and its structure Module Detail Subject Name Chemistry Course Name Chemistry 03 (Class XII, Semester 01) Mo
![Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why? from Chemistry Coordination Compounds Class 12 Assam Board Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why? from Chemistry Coordination Compounds Class 12 Assam Board](https://www.zigya.com/application/zrc/images/qvar/CHEN12070288.png)
Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why? from Chemistry Coordination Compounds Class 12 Assam Board
![Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty](https://www.wiredfaculty.com/application/zrc/images/qvar/CHEN12070315.png)
![PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2 PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2](https://i1.rgstatic.net/publication/256461217_Synthesis_structure_and_thermal_decomposition_of_NiNH3_6VOO22NH32/links/598c15b1458515c333a5e1ff/largepreview.png)
![Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby](https://content.bartleby.com/qna-images/answer/c73807b6-45ed-4420-a3b1-cee7bec70749/203ec893-0265-480e-b058-95e1378e904d/3cda3a30-aca8-11eb-99b8-c16e6153b0d2_IMG_20210504_124206_523~2.jpg)



![Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^( Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
![Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table](https://www.researchgate.net/publication/256461217/figure/tbl1/AS:667594596024340@1536178363556/Characteristic-bands-in-infrared-spectrum-of-NiNH3-6-VOO-2-2-NH-3-2.png)
![Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^( Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S01.png)