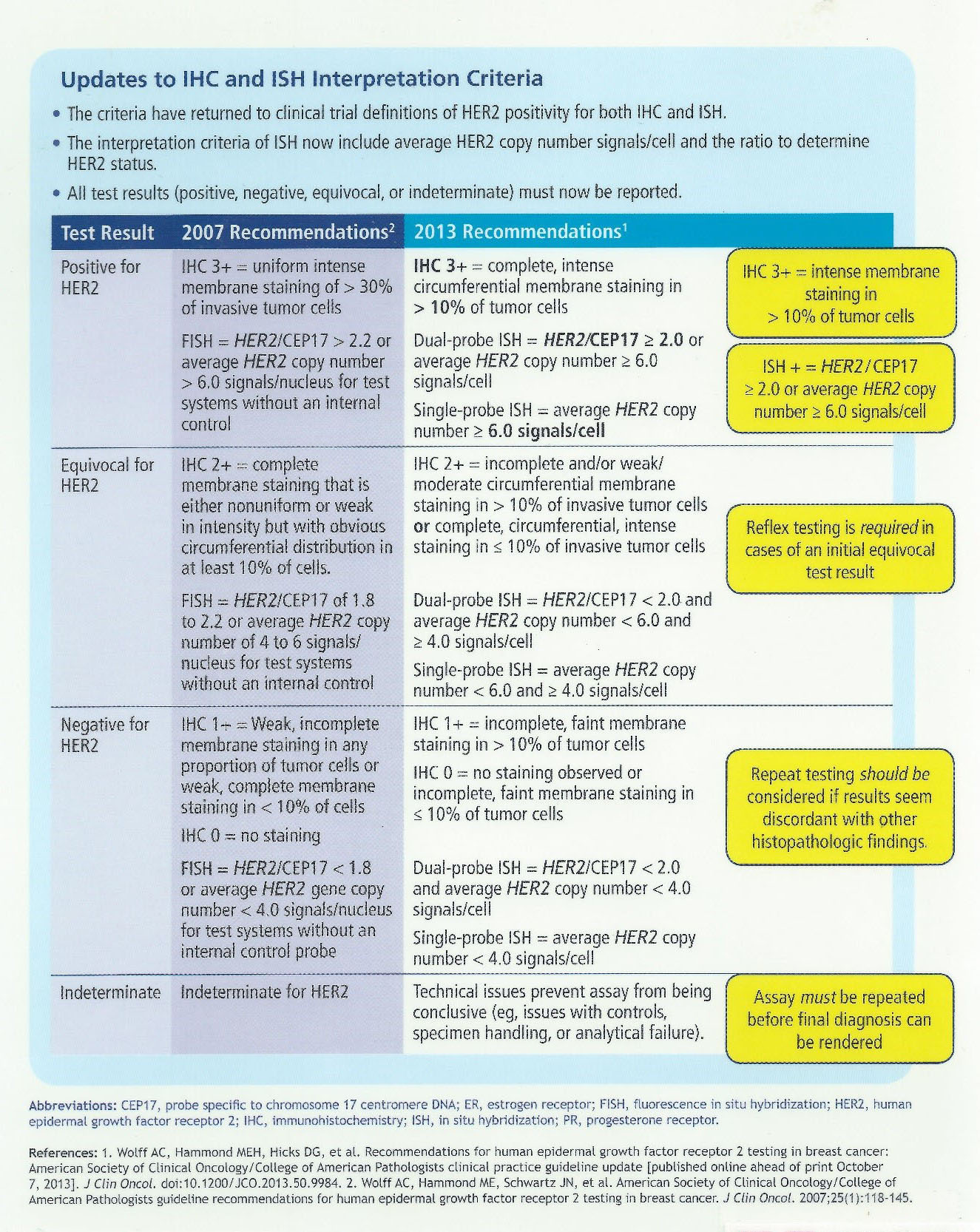
Comparison of the 2007 and 2013 ASCO/CAP evaluation systems for HER2 amplification in breast cancer - ScienceDirect
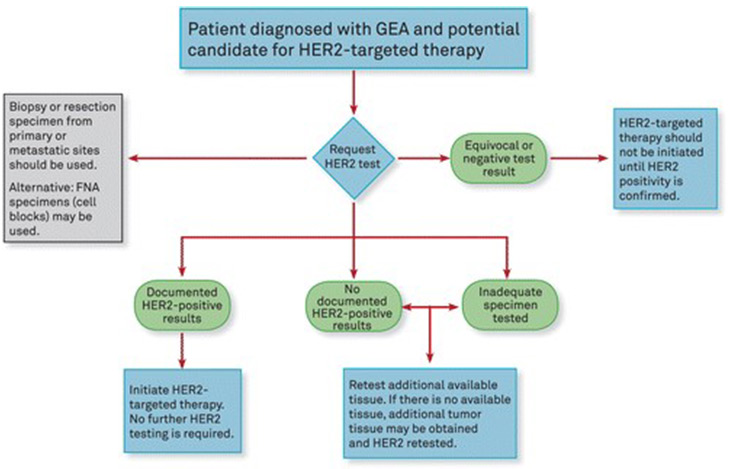
Frontiers | HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer
PDF) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer
![PDF] HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical PDF] HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical](https://d3i71xaburhd42.cloudfront.net/c7289407931d6ff274350f21b4024900edadc66e/2-Figure1-1.png)
PDF] HER2 Gene Amplification Testing by Fluorescent In Situ Hybridization (FISH): Comparison of the ASCO-College of American Pathologists Guidelines With FISH Scores Used for Enrollment in Breast Cancer International Research Group Clinical
![PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/22a3980f48cd672241df3eaefc79b28510b3576b/4-Figure1-1.png)
PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar
![PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/747f72db6685fa520b07391fb016bac6754d7633/9-Figure4-1.png)
PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar
![PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/22a3980f48cd672241df3eaefc79b28510b3576b/10-Figure2-1.png)
PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar
![PDF] Assessment of ERBB2/HER2 Status in HER2-Equivocal Breast Cancers by FISH and 2013/2014 ASCO-CAP Guidelines | Semantic Scholar PDF] Assessment of ERBB2/HER2 Status in HER2-Equivocal Breast Cancers by FISH and 2013/2014 ASCO-CAP Guidelines | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d99c6aa1dcc660fbabc1af5e20d607b8747a33a1/3-Figure1-1.png)
PDF] Assessment of ERBB2/HER2 Status in HER2-Equivocal Breast Cancers by FISH and 2013/2014 ASCO-CAP Guidelines | Semantic Scholar
![PDF] American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. | Semantic Scholar PDF] American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/32ece30ca42786761f6116717941ca1d4449b4bc/8-Figure1-1.png)
PDF] American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. | Semantic Scholar
Rate of reclassification of HER2-equivocal breast cancer cases to HER2-negative per the 2018 ASCO/CAP guidelines and response of HER2-equivocal cases to anti-HER2 therapy | PLOS ONE

Frontiers | Breast Cancer With a HER2 IHC2+ and FISH HER2/CEP17 Ratio ≥2.0 and an Average HER2 Gene Copy Number <4.0 per Tumor Cell: HER2 mRNA Overexpression Is a Rare Event
![PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/747f72db6685fa520b07391fb016bac6754d7633/8-Table2-1.png)
PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar

Effect of the 2013 ASCO-CAP HER2 Testing Guideline on the Management of IHC/ HER2 2+ Invasive Breast Cancer | Anticancer Research
![PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/747f72db6685fa520b07391fb016bac6754d7633/7-Figure1-1.png)
PDF] THE BOTTOM LINE Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer : American Society of Clinical Oncology / College of American Pathologists Clinical Practice Guideline Focused Update Guideline Questions | Semantic Scholar
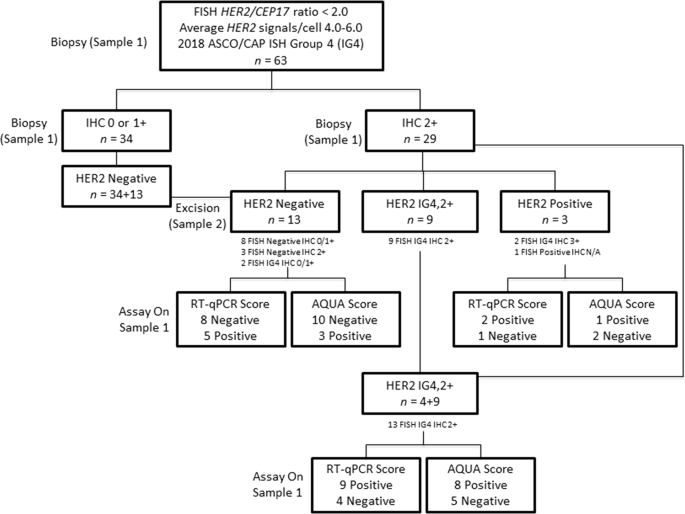
Quantitative assessments and clinical outcomes in HER2 equivocal 2018 ASCO/ CAP ISH group 4 breast cancer | npj Breast Cancer

PDF) Impact of modified 2013 ASCO/CAP guidelines on HER2 Testing in breast cancer. One year experience
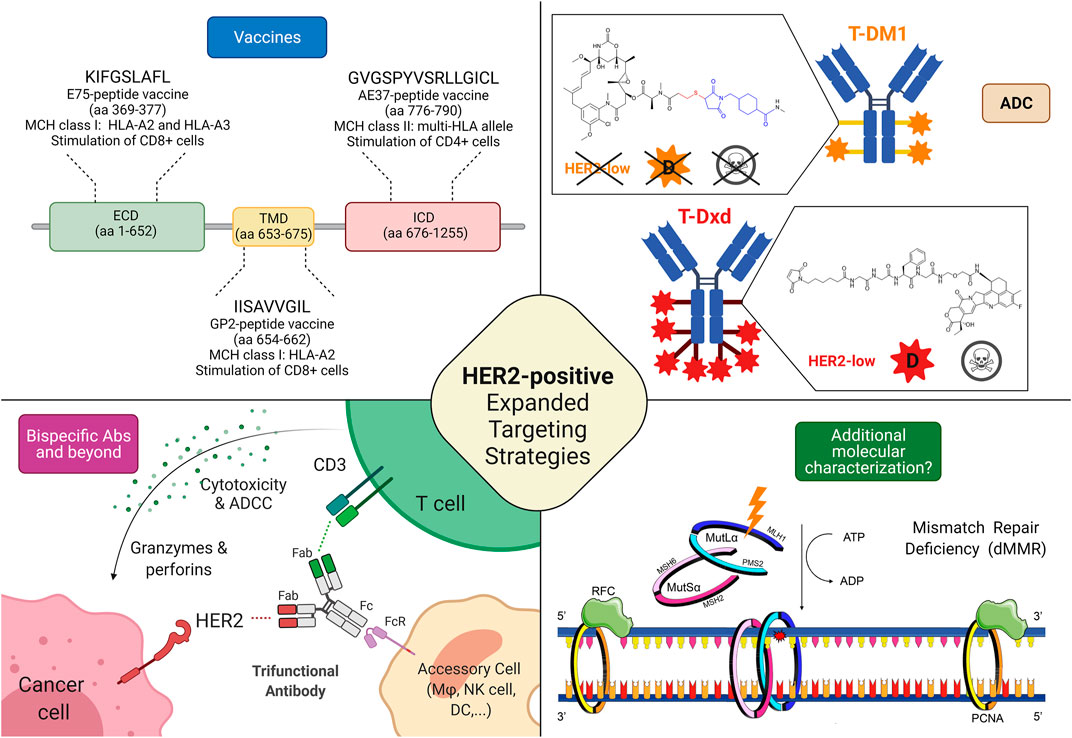
Frontiers | HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer
![PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar PDF] Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5b573aa3e76dd22869e0e5d0714a1a897b9647ef/22-Table1-1.png)