Safety and immunogenicity of the FINLAY-FR-1A vaccine in COVID-19 convalescent participants: an open-label phase 2a and double-blind, randomised, placebo-controlled, phase 2b, seamless, clinical trial - The Lancet Respiratory Medicine

Flowchart of screening for randomized clinical trials and for run-in... | Download Scientific Diagram

NIA-Funded Active Alzheimer's and Related Dementias Clinical Trials and Studies | National Institute on Aging

Characterisation of SARS-CoV-2 genomic variation in response to molnupiravir treatment in the AGILE Phase IIa clinical trial | Nature Communications
Results of the phase IIa RADICAL trial of the FGFR inhibitor AZD4547 in endocrine resistant breast cancer | Nature Communications

Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial - The Lancet

Early Phase Clinical Trial Solutions - CMIC | Pharmaceutical Development Services (CRO, CDMO, CSO, Healthcare, Japan Entry)
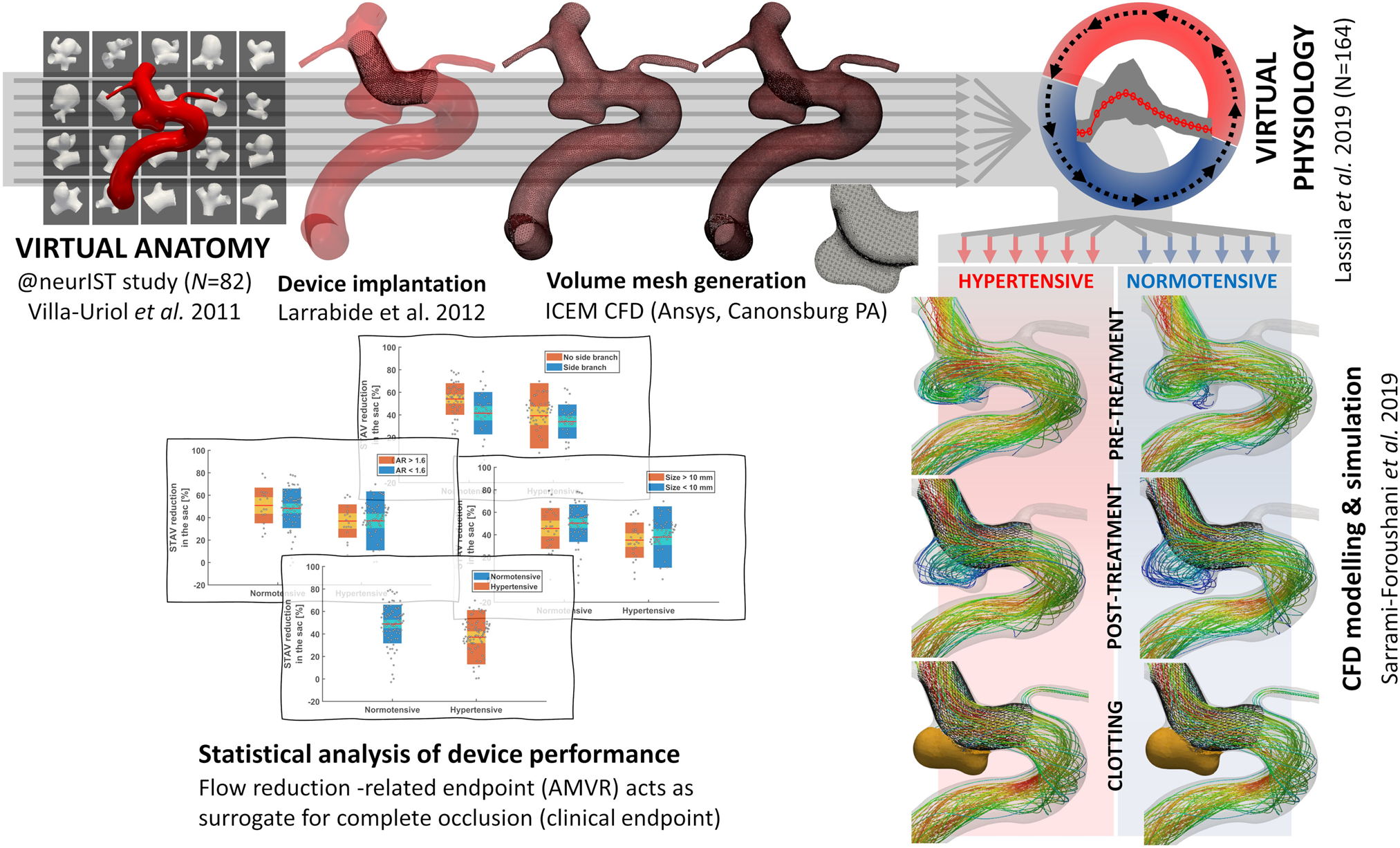
In-silico trial of intracranial flow diverters replicates and expands insights from conventional clinical trials | Nature Communications