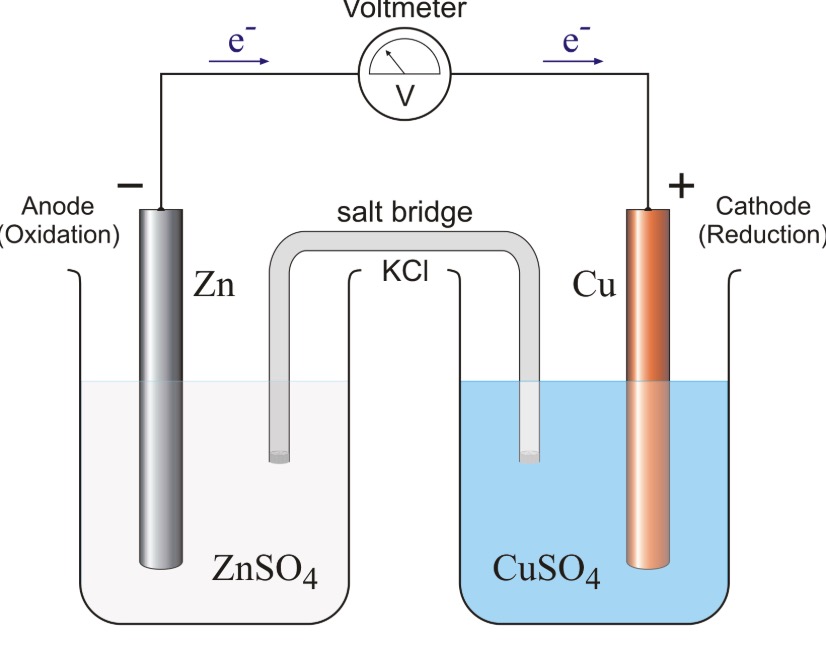
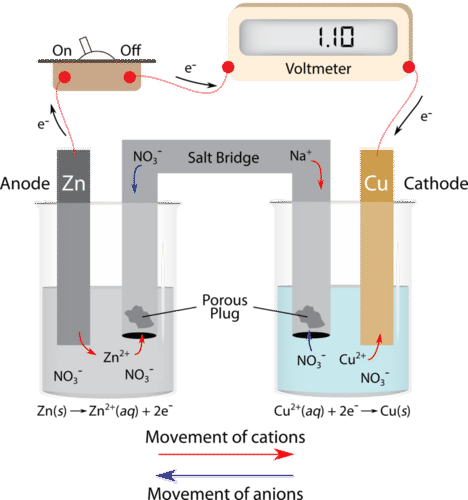
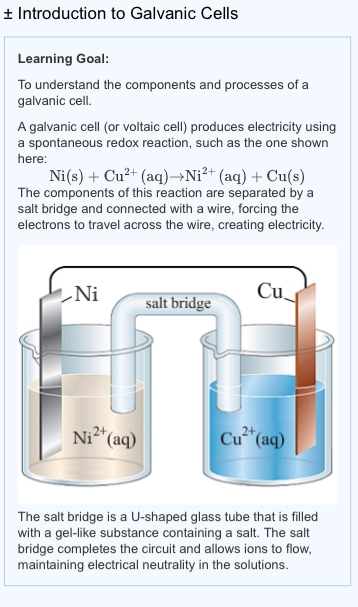
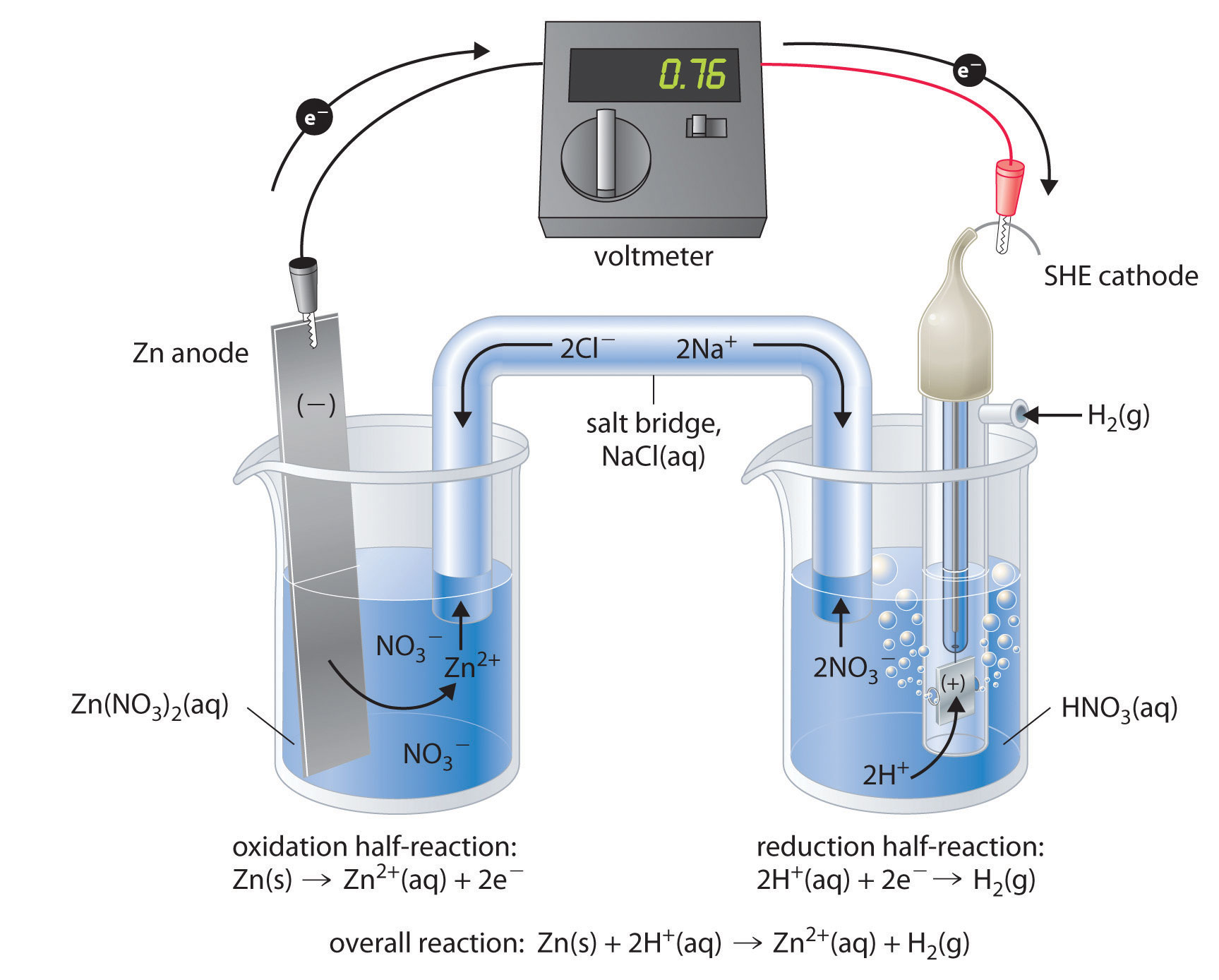
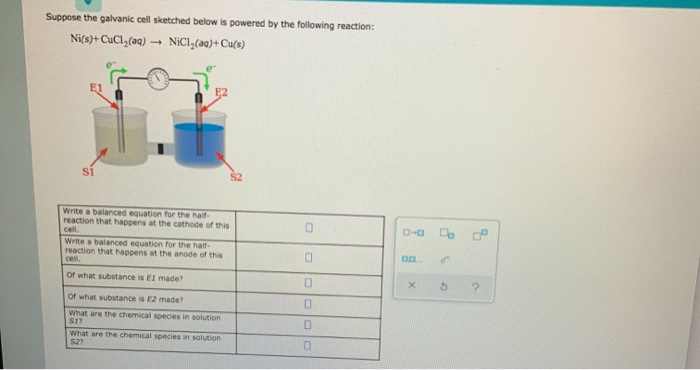
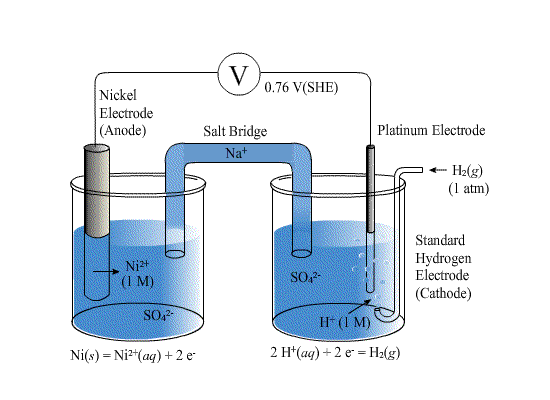
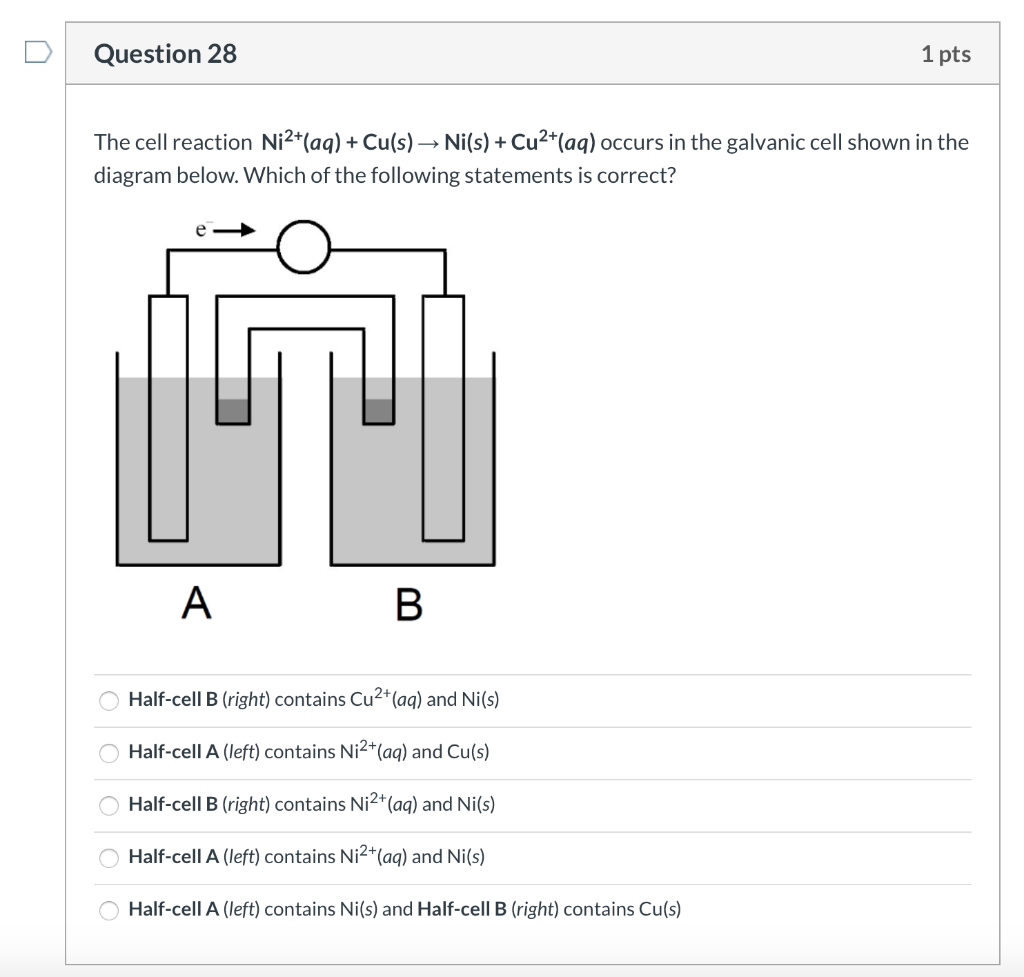
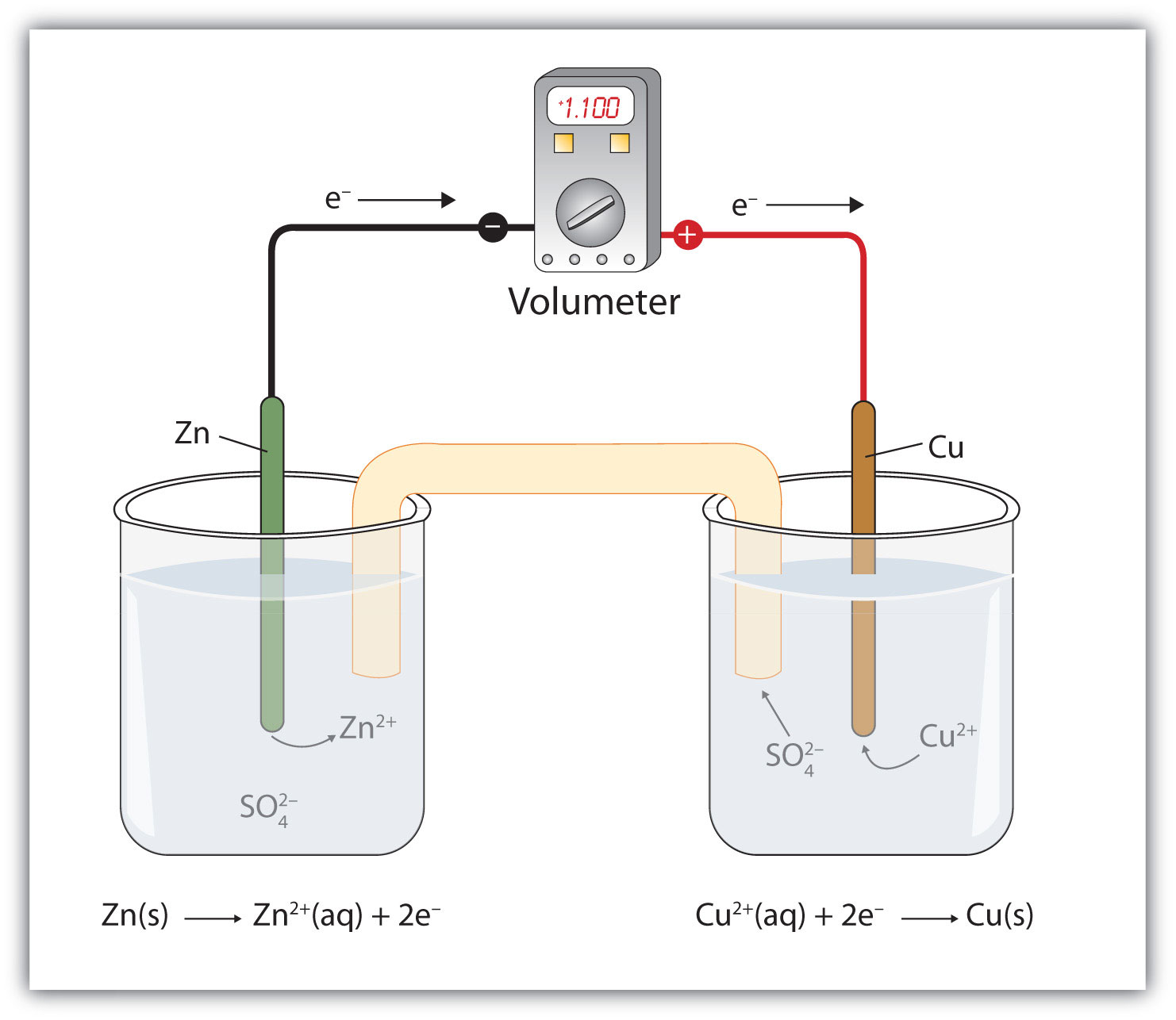
Mental model drawing for a given galvanic cell (Zn|Zn 2+ ||Cu|Cu 2+ ,... | Download Scientific Diagram

The EMF of the cell Ni `|Ni|Ni^(2+) ||Cu^(2+) |Cu(s)` is `0.59 ` volt. The standard reduction - YouTube

Example of a student generated Mg–Fe galvanic cell in the model kit,... | Download Scientific Diagram

A voltaic cell consists of a silver-silver ion half-cell and a nickel-nickel(II) ion half-cell. Silver ion is reduced during operation of the cell. Sketch the cell, labeling the anode and cathode and

Draw a picture of a voltaic electrochemical cell using Ni and Fe electrodes and 0.1 M NiCl_2 and FeCl_2 solutions. Be sure to label the following: anode, cathode, salt bridge, the metals,

Sketch a voltaic cell for this redox reaction: Ni^{2+} (aq) + Mg (s) to Ni (s) + Mg^{2 +}(aq) a. Label the anode and cathode. b. Write the half reactions. c. Indicate
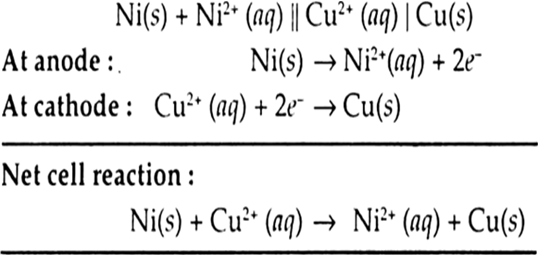
E0Ni2+/Ni and E0Cu2+/Cu are -0.25 V and + 0.34 respectively at 298 K. Formulate the self operating galvanic cell for this electrode pair. What reaction takes place in its operation? How is