Geometrical shapes of complexes formed by the reaction of ${\\text{N}}{{\\text{i}}^{{\\text{ + 2}}}}$ with ${\\text{C}}{{\\text{l}}^{\\text{ - }}}{\\text{,C}}{{\\text{N}}^{\\text{ - }}}$and ${{\\text{H}}_{\\text{2}}}{\\text{O}}$, respectively are:(A ...
![The geometry of \\[Ni{{(CO)}_{4}}\\]and \\[Ni{{(PP{{h}_{3}})}_{2}}C{{l}_{2}}\\]are :(A) Both square planar(B) Tetrahedral and square planar, respectively(C) Both tetrahedral(D) Square planar and tetrahedral, respectively The geometry of \\[Ni{{(CO)}_{4}}\\]and \\[Ni{{(PP{{h}_{3}})}_{2}}C{{l}_{2}}\\]are :(A) Both square planar(B) Tetrahedral and square planar, respectively(C) Both tetrahedral(D) Square planar and tetrahedral, respectively](https://www.vedantu.com/question-sets/26080d1f-f0b7-482a-b3ca-4dc1b6ce1eb64722761873556963438.png)
The geometry of \\[Ni{{(CO)}_{4}}\\]and \\[Ni{{(PP{{h}_{3}})}_{2}}C{{l}_{2}}\\]are :(A) Both square planar(B) Tetrahedral and square planar, respectively(C) Both tetrahedral(D) Square planar and tetrahedral, respectively
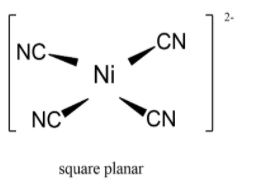
Why does [NiCl4]2- possess a tetrahedral geometry, whereas Ni(CN)4]2- possesses a square planar geometry? - Quora
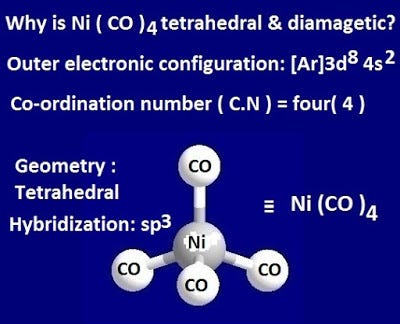
Why is Ni (CO) 4 tetrahedral and diamagnetic? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Square Planar vs Tetrahedral Coordination in Diamagnetic Complexes of Nickel(II) Containing Two Bidentate π-Radical Monoanions | Inorganic Chemistry
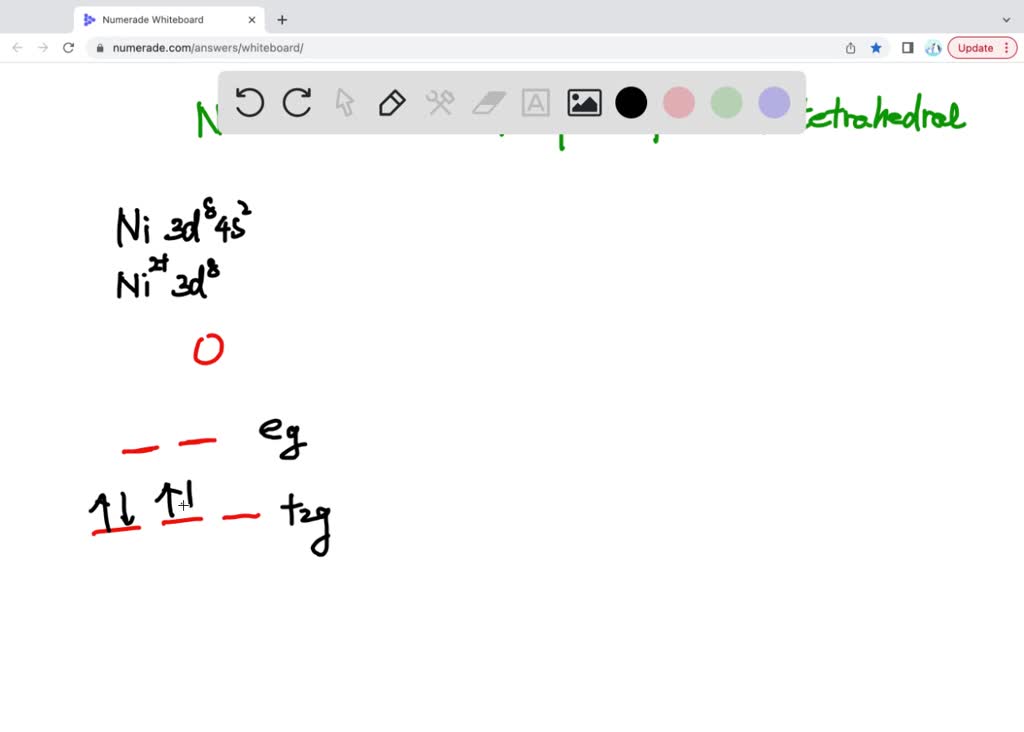
SOLVED: Nickel (II) forms octahedral, square planar and tetrahedral complexes with very different colours typically onserved. Draw the crystal field splitting diagrams showing:a) the electronic state of each of theseb) the electron
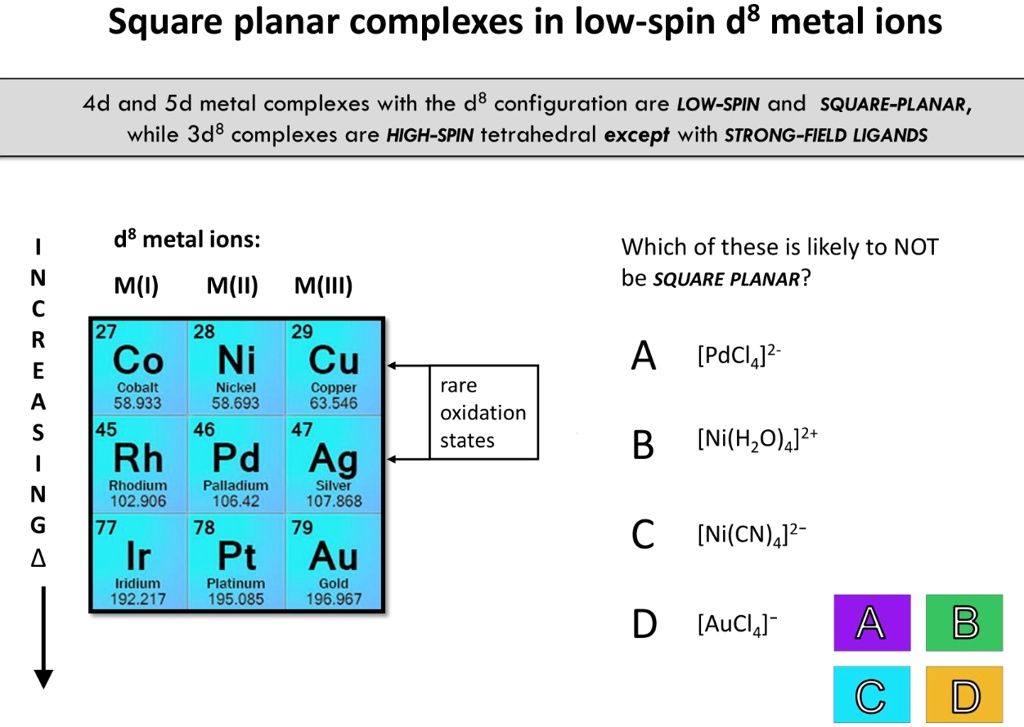
SOLVED: Square planar complexes in low-spin d8 metal ions 4d and Sd metal complexes with the d8 configuration are LOW-SPIN and SQUARE-PLANAR, while 3d8 complexes are HIGH-SPIN tetrahedral except with STRONG-FIELD LIGANDS

Square Planar vs Tetrahedral Coordination in Diamagnetic Complexes of Nickel(II) Containing Two Bidentate π-Radical Monoanions | Inorganic Chemistry

Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes
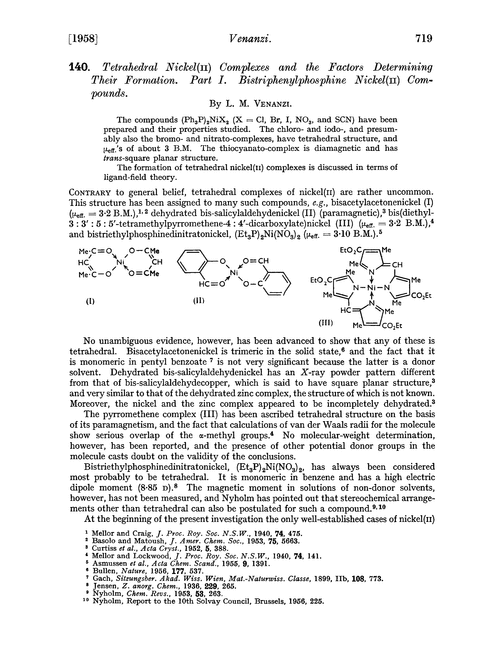
140. Tetrahedral nickel(II) complexes and the factors determining their formation. Part I. Bistriphenylphosphine nickel(II) compounds - Journal of the Chemical Society (Resumed) (RSC Publishing)

Suggested structure of the square planar Ni(II) complex of the ligand,... | Download Scientific Diagram
![Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why? from Chemistry Coordination Compounds Class 12 Rajasthan Board - English Medium Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why? from Chemistry Coordination Compounds Class 12 Rajasthan Board - English Medium](https://www.zigya.com/application/uploads/images/chen12070385-a_57148463d8ab9.png)
Ni(CO)4 has a tetrahedral geometry whereas [Ni(CN)4]4– has square planar geometry. Why? from Chemistry Coordination Compounds Class 12 Rajasthan Board - English Medium
![The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com](https://homework.study.com/cimages/multimages/16/cms53022462554618608855.jpg)
The diamagnetic [Ni(CN)_4]^2- ion has square-planar geometry, and the paramagnetic [NiCl_4]^2- ion has tetrahedral geometry. Use crystal field splitting diagrams to explain the difference in the magne | Homework.Study.com
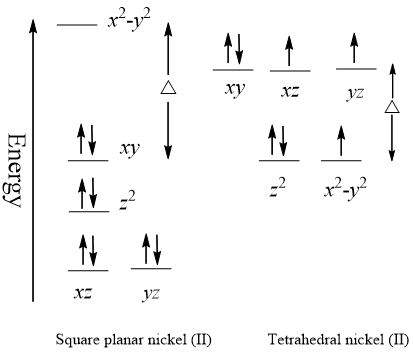
Solved: Chapter 20 Problem 120P Solution | Student Solutions Manual For General Chemistry 2nd Edition | Chegg.com

![coordination compounds - Why is Ni[(PPh₃)₂Cl₂] tetrahedral? - Chemistry Stack Exchange coordination compounds - Why is Ni[(PPh₃)₂Cl₂] tetrahedral? - Chemistry Stack Exchange](https://i.stack.imgur.com/AD72m.png)
![Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube Ni(CO)4, [Ni(CN)4]2-, [NiCl4]2-Structure-Hybridization-VBT-IIT JEE NEET SAT NCERT CBSE - YouTube](https://i.ytimg.com/vi/r_C4yyTUSjM/maxresdefault.jpg)


![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-1.png)
