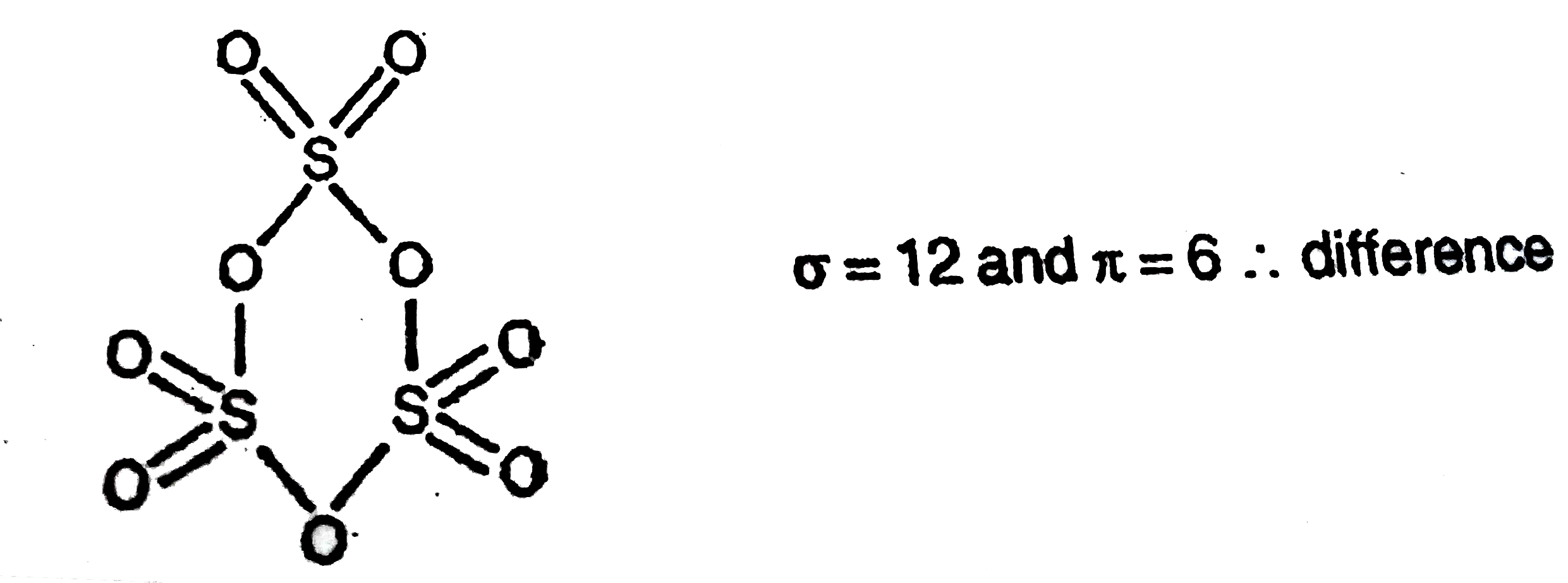
Welcome to Chem Zipper.com......: What are the structure of cyclic trimer of SO3 , Methylcyclotrisilicone, Cyclotrimetaphosphate and cyclotrisilicate ion ?

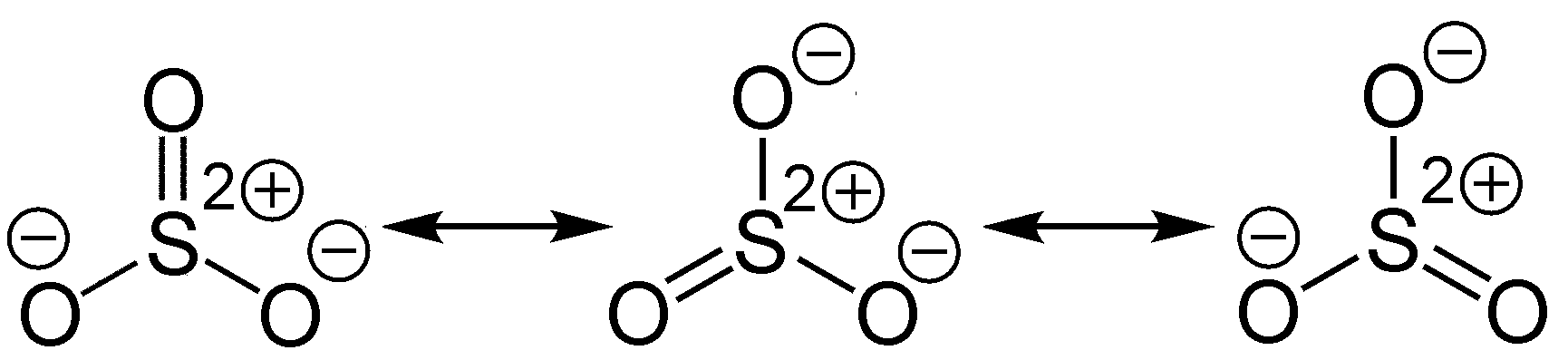
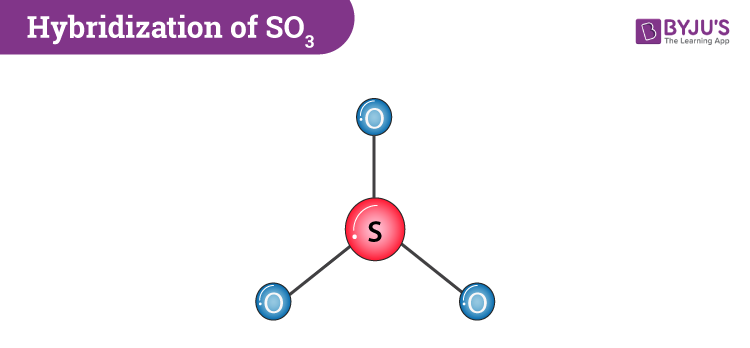
Welcome to Chem Zipper.com......: What are the number of sigma bond, pi-bond and lone pair in the lewis dot structure of SO3 ?

In the LEWIS Structure of SO3, the number of sigma and pi bonds respectively are 3, 3 3, 2 - Chemistry - The Solid State - 14954939 | Meritnation.com
![Describe the bonding in SO2 and SO3 using the localized electron model (hybrid orbital theory). How would the molecular orbital model describe the [{MathJax fullWidth='false' \pi }] bonding in these two compounds? Describe the bonding in SO2 and SO3 using the localized electron model (hybrid orbital theory). How would the molecular orbital model describe the [{MathJax fullWidth='false' \pi }] bonding in these two compounds?](https://homework.study.com/cimages/multimages/16/screenshot_2022-12-02_1257511014901097068014803.png)